Pharmaceutical Rapid Microbiology Testing Market Size to Surpass USD 5.64 Billion by 2034
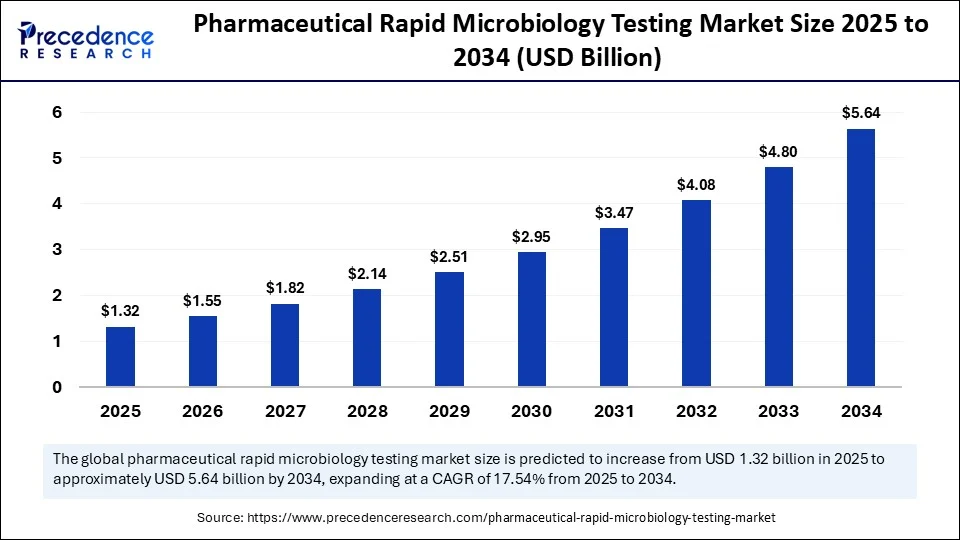
The pharmaceutical rapid microbiology testing market shows From USD 1.12 billion in 2024 to USD 5.64 billion by 2034 with a strong CAGR of 17.54%.
Pharmaceutical Rapid Microbiology Testing Market Key Takeaways
- Pharmaceutical Rapid Microbiology Testing Market to grow from USD 1.12 billion in 2024 to USD 5.64 Billion by 2034.
- The market is expected to grow at a CAGR of 17.54% from 2025 to 2034.
- North America leads with 38%; Asia Pacific fastest-growing
- Top tech: PCR; fastest-growing tech: flow cytometry
- Top product: Instruments; fastest-growing: software & services
- Top application: Biopharmaceuticals; rising: vaccines
- Most tested: Bacteria; rising: viruses
- Top end-user: Pharma & biotech firms; fastest: CMOs
- Top test: Sterility; rising: environmental monitoring
How is Artificial Intelligence Enhancing the Efficiency of Pharmaceutical Rapid Microbiology Testing?
Artificial intelligence is reshaping the pharmaceutical rapid microbiology testing landscape. From improving data accuracy and speeding up analysis to enabling real-time decision-making, AI is taking center stage. It streamlines lab operations—automating tasks like colony counting—and empowers teams to detect microbial threats faster and more reliably.
As pharma companies scale up biologics and sterile drug production, avoiding contamination is critical. That’s why AI-driven tools, combined with smart automation and biosensors, are becoming a must-have. With global markets prioritizing efficiency and quality, the demand for intelligent RMT solutions is only going to rise.
Get a Free Sample Copy of the Report@ https://www.precedenceresearch.com/sample/6332
Market Overview
The pharmaceutical rapid microbiology testing market is undergoing a technological transformation, with a focus on precision, scalability, and regulatory compliance. Rapid microbiological methods are increasingly being adopted in pharmaceutical quality assurance processes, particularly in the manufacturing of sterile and biologic products where microbial contamination risks are high. These methods offer significant advantages, including reduced testing time, lower risk of contamination, and higher sensitivity and specificity.
Drivers
The increasing global burden of microbial contamination in drug production and the simultaneous demand for faster drug release cycles are primary drivers. Regulatory bodies are encouraging pharmaceutical companies to adopt rapid microbiology systems to meet current Good Manufacturing Practices (cGMP) standards. Automation, artificial intelligence, and miniaturization of assay platforms are making these technologies more accessible and reliable.
Opportunities
There is growing potential in integrating predictive analytics and cloud-based laboratory information systems into rapid microbiology platforms. These integrations can enable remote monitoring, data sharing, and predictive quality control. Additionally, emerging biotechnology startups are developing low-cost rapid testing kits that are especially suited to resource-limited settings, creating a unique expansion opportunity in underserved markets.
Challenges
Challenges facing the pharmaceutical rapid microbiology testing market include regulatory complexities related to method validation, and a fragmented landscape of standards across regions. High operational costs and the learning curve associated with new technologies can discourage smaller firms from early adoption. Maintaining data integrity and compliance in automated environments also requires significant oversight.
Regional Insights
The United States remains a frontrunner due to its advanced pharmaceutical infrastructure and early adoption of innovative testing technologies. The European Union follows with strong enforcement of GMP and focus on aseptic manufacturing. Asia Pacific presents a high-growth opportunity due to rising pharmaceutical exports, supportive government schemes, and improved laboratory capabilities, especially in India, China, and South Korea.
Pharmaceutical Rapid Microbiology Testing Market Companies

- Thermo Fisher Scientific Inc.
- Merck KGaA (MilliporeSigma)
- Bio-Rad Laboratories, Inc.
- Lonza Group AG
- Charles River Laboratories International, Inc.
- 3M Company
- Hamilton Company
- Microbiological Solutions (a part of Pall Corporation)
- Pall Corporation
- Pall Life Sciences
- Qiagen N.V.
- PerkinElmer, Inc.
- Eurofins Scientific
- IDEXX Laboratories, Inc.
- BioMérieux SA
- Neogen Corporation
- Agilent Technologies, Inc.
- Nova Biomedical Corporation
- LuminUltra Technologies Ltd.
- Accelerate Diagnostics, Inc.
Recent Developments
Recent innovations include the deployment of AI-enabled microbial detection systems that automate both identification and reporting. Global manufacturers are also investing in portable RMT devices suitable for decentralized facilities. Research partnerships between academia and industry are accelerating the development of novel biosensors and microfluidic testing platforms, which promise to redefine microbial quality assurance in pharmaceuticals.
Rapid Infection Diagnostics Inc. launched its BSIDx system on January 16, 2025, slashing bloodstream infection testing time to under 5 hours.
- On March 4, 2025, Nelson Labs began rapid sterility testing at sites in the U.S. and Germany, reducing incubation from 14 to 6 days while staying USP <71> compliant.
- On April 22, 2025, Redberry validated a 4-day sterility test, cutting standard release timelines in half with full regulatory compliance
Segments Covered in the Report
By Technology Type
- Polymerase Chain Reaction (PCR)
- ATP Bioluminescence
- Flow Cytometry
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Biosensors & Bioassays
- Impedance Microbiology
- Others (e.g., Microfluidics, Microarray)
By Product Type
- Instruments
- Reagents & Consumables
- Software & Services
By Application
- Biopharmaceuticals
- Small Molecule Pharmaceuticals
- Vaccines
- Contract Research Organizations (CROs)
- Academic & Research Institutes
By Microorganism Type Tested
- Bacteria
- Fungi (Yeast and Mold)
- Viruses
- Mycoplasma
- Endotoxins
By End User
- Pharmaceutical & Biotechnology Companies
- Contract Manufacturing Organizations (CMOs)
- Hospitals & Diagnostic Laboratories
- Research & Academic Institutes
By Testing Type
- Sterility Testing
- Environmental Monitoring
- Bioburden Testing
- Raw Material Testing
- Water Testing
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Ready for more? Dive into the full experience on our website!
https://www.precedenceresearch.com/
