Battery Free Implants Market Size to Surpass USD 43.55 Billion by 2034
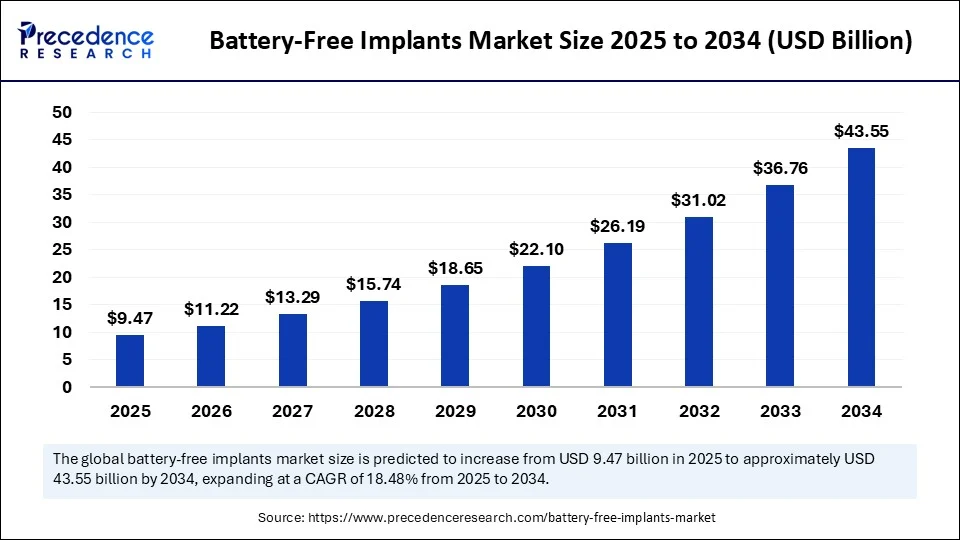
The global battery free implants market size is valued to reach around USD 43 55 billion by 2034 increasing from USD 7 99 billion in 2024, with a CAGR of 18.48%.
Battery Free Implants Market Key Takeaways
- In terms of revenue, the global battery-free implants market was valued at USD 7.99 billion in 2024.
- It is projected to reach USD 43.55 billion by 2034.
- The market is expected to grow at a CAGR of 18.48% from 2025 to 2034.
- North America dominated the global battery-free implants market in 2024.
- Asia Pacific is expected to grow at the fastest CAGR from 2025 to 2034.
- By application, the cardiac monitoring and pacing devices segment held the major market share in 2024.
- By application, the orthopedic monitoring devices segment is projected to grow at the highest CAGR between 2025 and 2034.
- By therapeutic area, the cardiology segment contributed the biggest market share in 2024.
- By therapeutic area, the orthopedics segment is expanding at a significant CAGR between 2025 and 2034.
- By energy harvesting technology, the radiofrequency (RF)-based devices segment led the market in 2024.
- By energy harvesting technology, the piezoelectric energy conversion segment is expected to grow at a significant CAGR over the projected period.
- By material, composite materials generated the largest market share in 2024.
- By material, bioresorbable materials segment is expected to grow at a notable CAGR from 2025 to 2034.
- By end user, the hospitals and clinics segment held the major market share in 2024.
- By end user, the homecare settings segment is projected to grow at a significant CAGR between 2025 and 2034
Impact of Artificial Intelligence on the Battery-Free Implants Market
AI is transforming the design and implementation of battery-free implants by enabling intelligent, swift, and accurate integration into clinical workflows. It enhances energy harvesting—harnessing body heat and movement—through AI-based modeling and optimization, ensuring reliable operation even in extreme biological environments.
In parallel, clinical AI analytics help select the best patient candidates for this technology. Moreover, AI is spearheading the development of more sustainable, less invasive, and highly personalized implantable electronics by minimizing battery usage and maximizing overall functionality
Get Sample Link@ https://www.precedenceresearch.com/sample/6346
Market Overview
The battery‑free implants market focuses on medical implants that function indefinitely without internal power sources. These devices harness ambient biological or external energy (thermal gradients, body movement, blood glucose, inductive RF, ultrasound) to perform functions such as monitoring physiological signals, delivering therapy, pacing the heart, or stimulating nerves.
By eliminating batteries, such implants reduce surgical replacement, enable ultra‑small footprints, and ultimately improve safety, patient comfort, and long-term healthcare efficiency. Recent progress in nanotechnology, wireless power transmission, and biocompatible materials has transformed these concepts into real-world prototypes and early clinical use cases.
Drivers
-
Chronic disease prevalence & aging trend: Increased global burden of heart disease, Parkinson’s, diabetes, and neurological disorders—especially among aging populations—drives need for maintenance-free implantable care devices.
-
Preference for less invasive and home‑based health delivery: Healthcare systems are pushing care closer to home; battery‑free implants reduce procedural risk and repeated interventions.
-
Technological maturation: Miniaturized circuits, efficient piezoelectric/triboelectric/thermoelectric harvesters, biofuel cells, and RF energy transfer systems provide viable power solutions without invasive recharging.
-
Telemedicine & remote analytics demands: Implantable devices that interface with remote systems enable continuous data flow, proactive healthcare, and AI‑driven response.
-
Supportive regulation and funding: National agencies increasingly support low‑invasion medical devices; grants and regulatory fast‑tracks are accelerating development and approval.
Opportunities
-
Adaptive clinical treatment systems: Devices that sense physiological states and adjust therapies (e.g. neuromodulation output) dynamically can deliver more precise treatment, enhancing outcomes.
-
Cross‑sector collaborations: Partnerships between medtech firms, semiconductor manufacturers, academic research institutions and energy‑harvesting innovators drive shared IP and manufacturing scale.
-
Exploration of new application areas: Beyond heart or neural implants, battery‑free technology offers potential in fertility sensing, ophthalmology, drug delivery implants, gastrointestinal monitoring, and force sensing in orthopedics.
-
Biodegradable/regenerative implant opportunities: Dissolvable implants reduce long‑term infection risks, surgical burdens, and appeal to transient diagnostic or pediatric use cases.
-
Geographic expansion into new markets: Developing nations with high disease burdens but limited surgical capacity could adopt battery‑free implants for long-term care without infrastructure-heavy maintenance.
Challenges
-
Unstable power harvesting: Biological conditions are variable; motion, heat or glucose harvested may not always meet power demands reliably across patient activities or health states.
-
Power limits for demanding applications: Devices performing therapeutic stimulation or deep implantation often require power beyond current harvest capacities.
-
High production and R&D costs: Precision micro‑fabrication, clinical validation, biocompatible materials and extended trials contribute to steep development costs and high product prices.
-
Lack of long‑term clinical validation: Long-term, peer-reviewed studies spanning multiple years remain limited, delaying broad adoption by clinicians and insurers.
-
Complex regulatory landscape & wireless risk: Varying international standards for energy emissions, electromagnetic interference, cybersecurity, and device integration complicate global deployment.
-
Cybersecurity exposure: Wireless implants connected to external networks or clinical systems can be vulnerable to breach unless robust security frameworks are in place.
Recent Developments
-
A cutting-edge micro‑pacemaker developed via additive manufacturing surfaced—just 4 mm in diameter, lead‑free and using ambient energy harvested internally, signaling a leap toward real-world battery‑free cardiac devices.
-
High‑resolution micropatterned circuits using liquid‑metal composites and RF coils have demonstrated energy harvesting up to 178 mW/cm², applicable to implantable optogenetic systems.
-
Metamaterial implants have achieved all‑mechanical wireless force sensing in orthopedic prototypes—no electronics required—offering a new paradigm for passive implantable sensors.
-
Advanced MXene‑based bioelectronic films permit low‑impedance, wireless, battery‑free cardiac sensing and stimulation—validated in animal models.
-
Passive ultrasonic communication links now allow implants deeper within tissue to transmit data without onboard electronics, using piezoelectric transducers.
-
Regulatory bodies (e.g. U.S. FDA, EU MDR) are actively shaping guidance around wireless, battery‑free implants—addressing thermal safety, cybersecurity, implant-monitor interoperability and biocompatible materials.
Battery-Free Implants Market Companies
- Abbott Laboratories
- Biotronik SE & Co. KG
- Cochlear Limited
- EBR Systems, Inc.
- Medtronic plc
- NeuroPace Inc.
- Pixium Vision
- Profusa Inc.
- Second Sight Medical Products Inc.
- Stimwave Technologies Inc.
Latest Announcement by Industry Leader
- In April 2025, CELTRO, established in late 2019, is a forward-thinking startup formed by heart rhythm specialists and experts from the semiconductor industry, including seasoned engineers and executives. The company strives to transform the integration of implants within the human body by designing electronic devices that function independently of external power sources. CELTRO’s inaugural initiative involves the development of a 3D-printed, battery-free pacemaker implant utilizing advanced additively manufactured electronics (AME) technology. “We are extremely excited by the progress we’ve made in the development and testing of our battery-free cardiac implant that has been enhanced using Nano Dimension’s AME technology. We are optimistic about the prospects of creating a platform of next-generation in-body electronics that will help shape the future of the medical implant industry” – Dr.-Ing Gerd Teepe, CELTRO Co-Founder & CEO.
Segments Covered in the Report
By Application
- Neural Stimulation Devices
- Cardiac Monitoring & Pacing Devices
- Drug Delivery Systems
- Bio-sensing and Diagnostics
- Hearing Implants
- Orthopedic Monitoring Device
By Therapeutic Area
- Cardiology
- Neurology
- Orthopedics
- Endocrinology (e.g., Glucose Monitoring)
- ENT (Ear, Nose, Throat)
- Urology & Gastroenterology
By Energy Harvesting Technology
- Radiofrequency (RF)-Based Devices
- Ultrasound Energy Harvesting
- Piezoelectric Energy Conversion
- Magnetic Resonance Coupling
- Thermoelectric & Bioelectric Harvesting
By Material Type
- Biocompatible Polymers
- Titanium & Other Metals
- Ceramic-Based Materials
- Bioresorbable Materials
- Composite Materials
By End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Research & Academic Institutes
- Homecare Settings
- Specialty Clinics
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
Also Visit@https://www.precedenceresearch.com/
